WO2014165660A1 - Pharmaceutical formulations for subcutaneous administration of furosemide - Google Patents

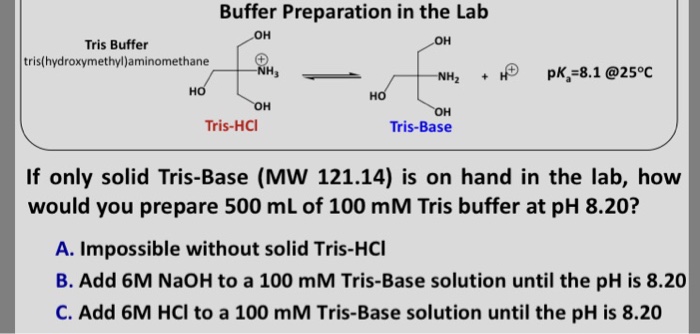
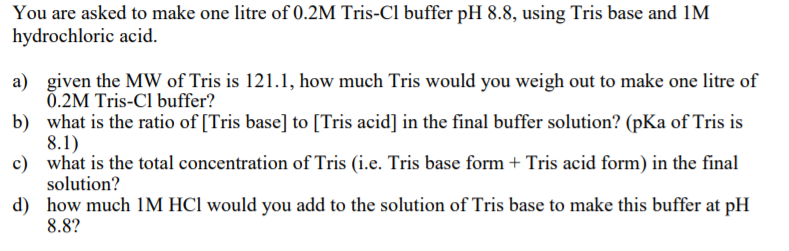
Tris(hidroximetil)-aminometano TRIS base is useful in the pH range of 7.0-9.0. Has a pKa of 8.1 at 25°C. | Sigma-Aldrich

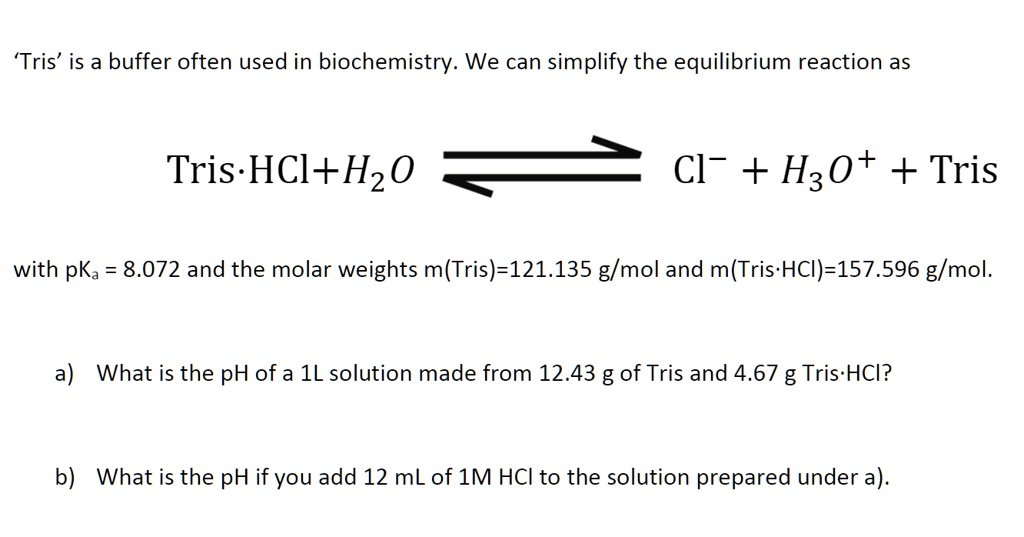
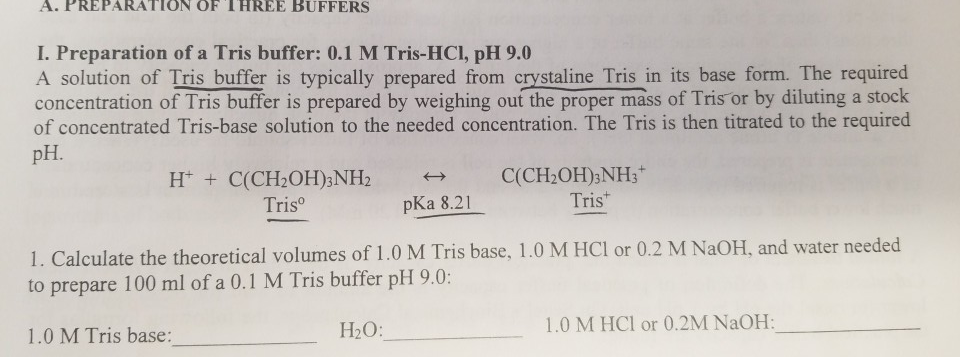
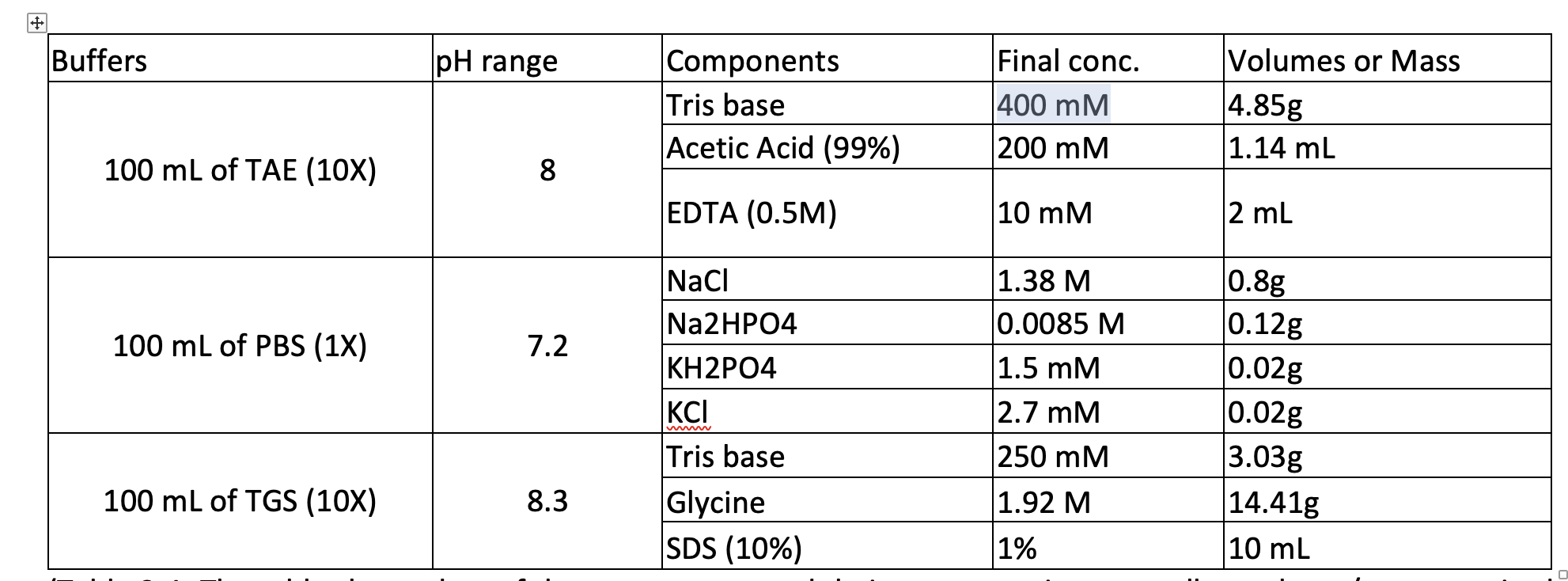
SOLVED: Solution #1: 500. mL of 150 mM Tris, pH 8.50 You have the following available Tris base (FW = 121.1 g/mol) pKa 8.10) 1.0 M HCI Solution #2: 750. mL of









![G-Biosciences TE BUFFER [1X], PH 8.0, LOW EDTA (TRIS-EDTA; 10MM TRIS BASE, | Fisher Scientific G-Biosciences TE BUFFER [1X], PH 8.0, LOW EDTA (TRIS-EDTA; 10MM TRIS BASE, | Fisher Scientific](https://assets.fishersci.com/TFS-Assets/CCG/product-images/VN00037630-786-150.JPG-650.jpg)
-500x500.jpg)