Mechanism of the Deprotonation Reaction of Alkyl Benzyl Ethers with n‐ Butyllithium - Raposo - 2013 - Chemistry – A European Journal - Wiley Online Library

n-Butyllithium, 1.6M solution in hexanes, AcroSeal , Thermo Scientific Chemicals, Quantity: 100 mL | Fisher Scientific

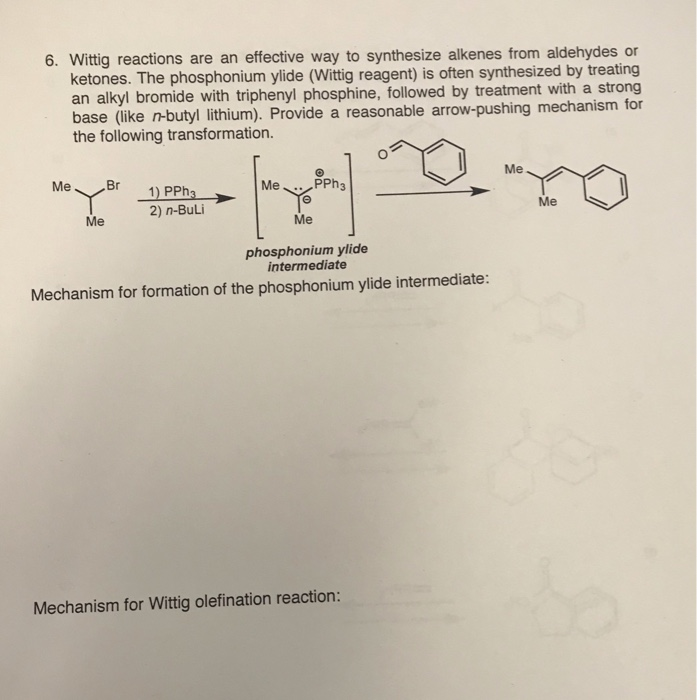
Lithium diisopropylamide is a strong base and nonnucleophilic base. It is often freshly prepared by treating a certain reactant with n-butyllithium (n -BuLi). Draw the starting material and draw the product (lithium diisopropylamide).

Grignard Reaction Key features: Handling of air/ moisture sensitive chemicals, formation of C-C bond. n-Butyl lithium Key features: Strong base such as. - ppt download
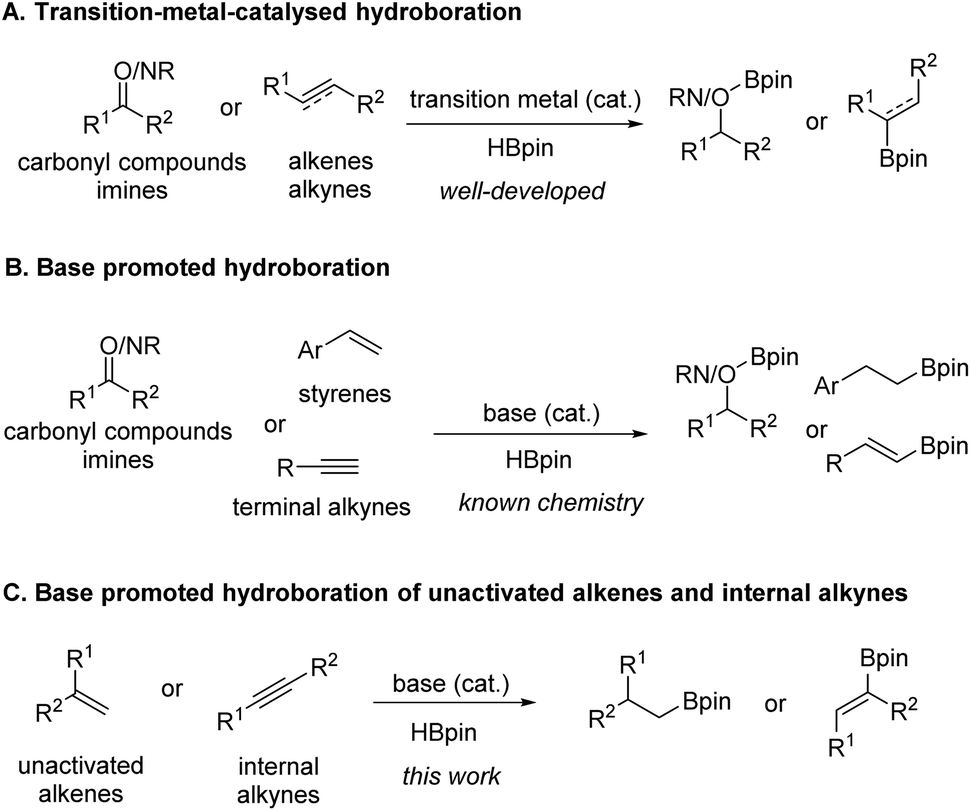
n BuLi-promoted anti -Markovnikov selective hydroboration of unactivated alkenes and internal alkynes - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C9QO00750D

n‐Butyllithium (1 mol %)‐catalyzed Hydroboration of Aldehydes and Ketones with Pinacolborane - Yang - 2019 - Bulletin of the Korean Chemical Society - Wiley Online Library

n-Butyllithium/N,N,N',N'-Tetramethylethylenediamine-Mediated Ortholithiations of Aryl Oxazolines: Substrate-Dependent Mechanisms | Journal of the American Chemical Society