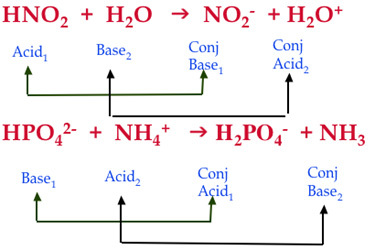
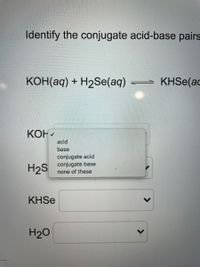
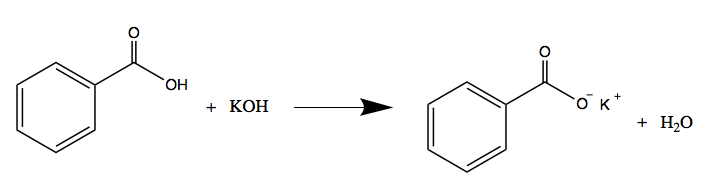
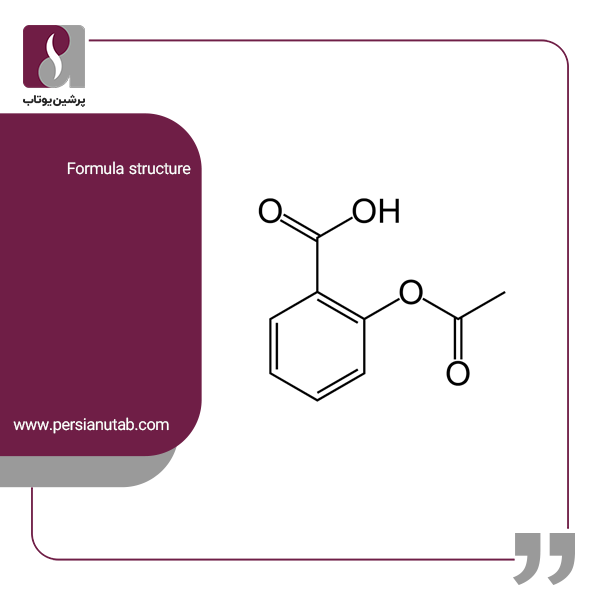
Question Video: Calculating the Concentration of Nitric Acid via Titrating against a Known Volume of Potassium Hydroxide | Nagwa
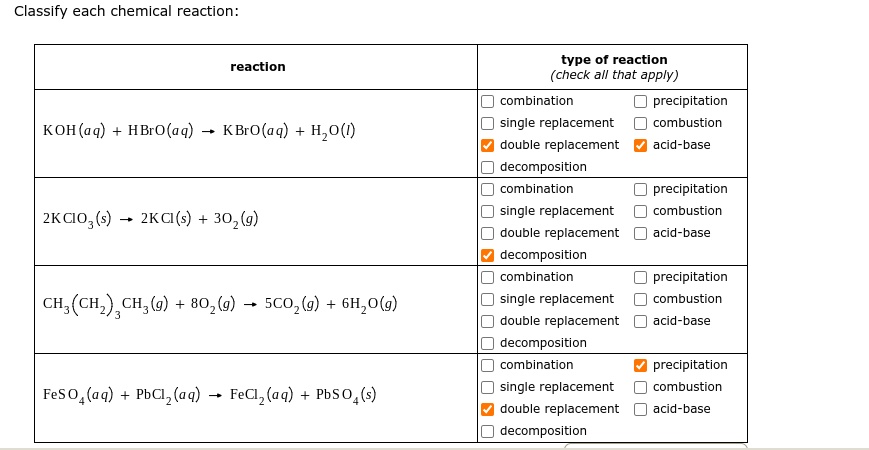
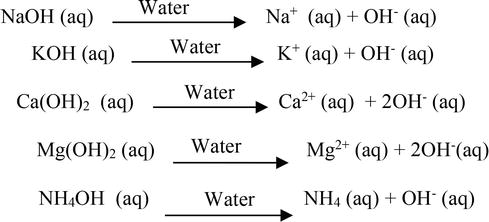
SOLVED: Classify each chemical reaction: type of reaction (check all that apply) combination precipitation reaction KOH(aq) + HBrO(aq) KBrO(aq) Hzo() single replacement double replacement combustion acid-base decomposition combination precipitation ...

In which of the following pair of reactions first reaction is spontaneous while second reaction is non spontaneous?
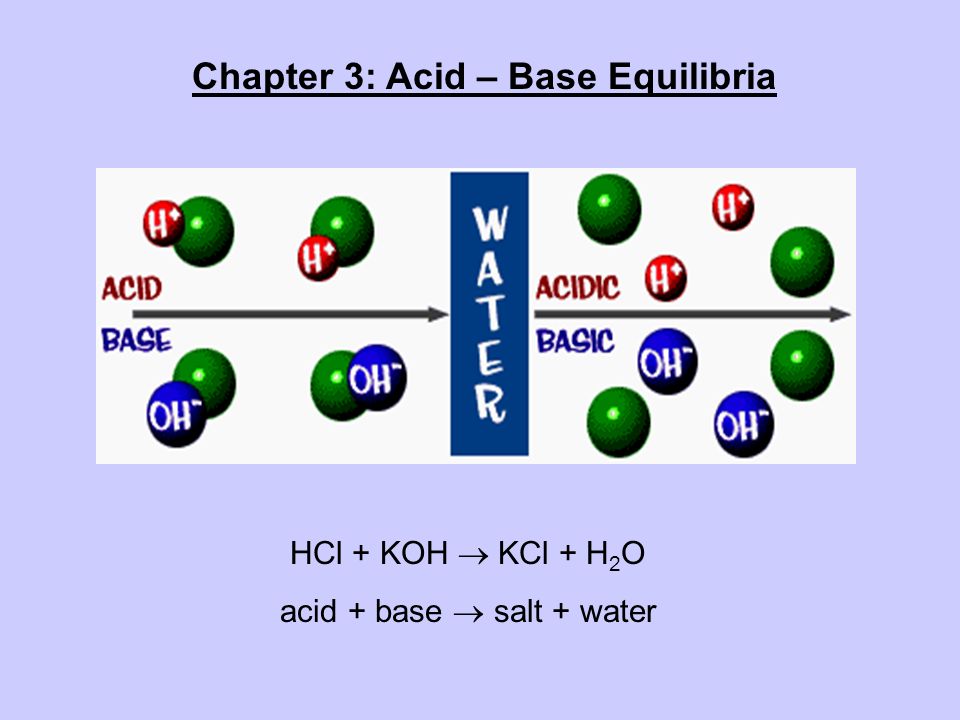
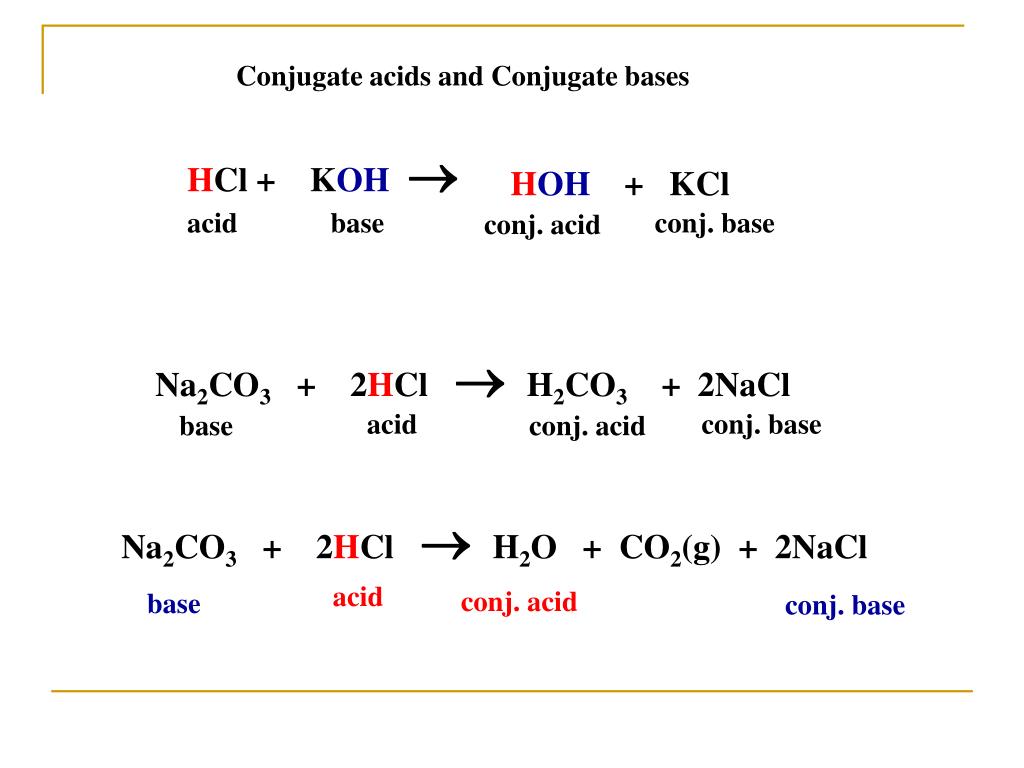
Chapter 3: Acid – Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water. - ppt download