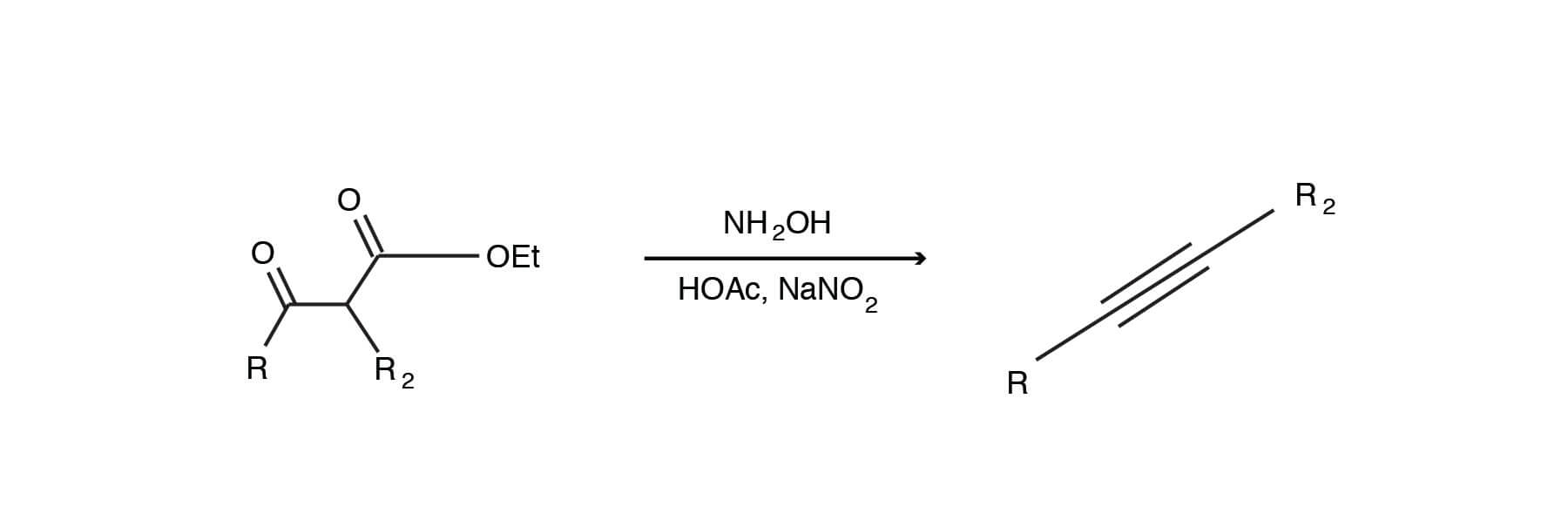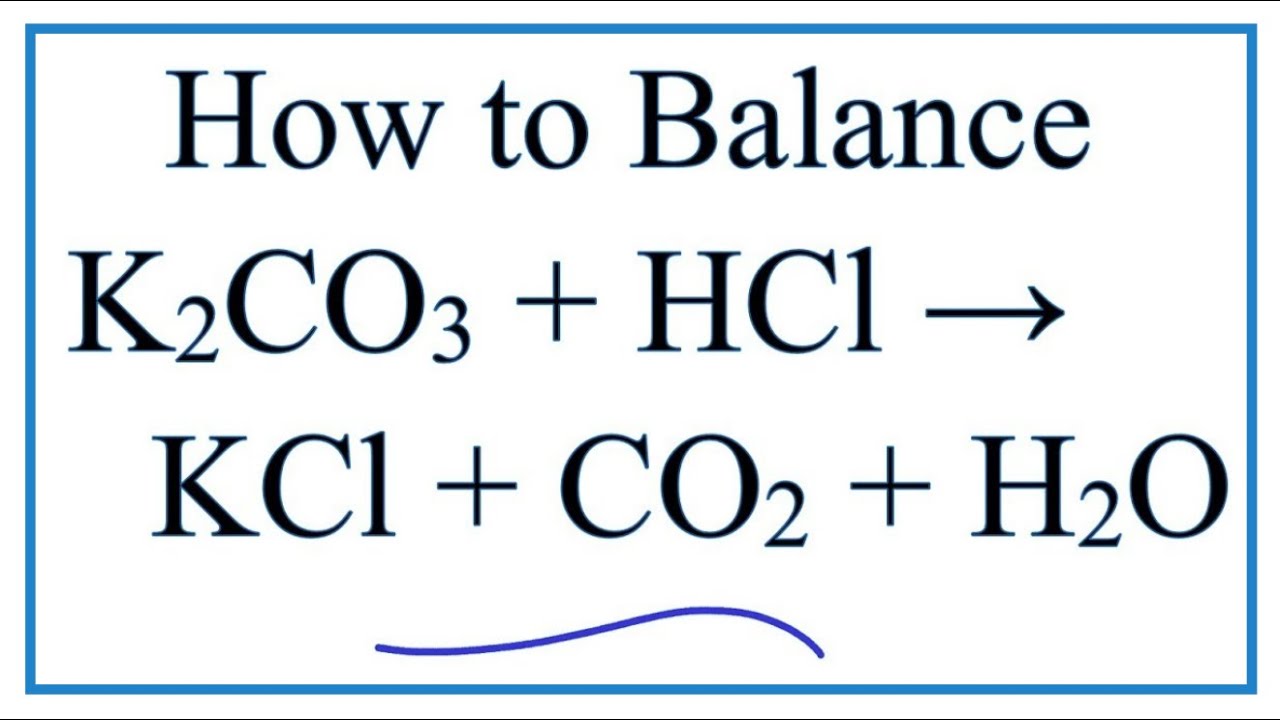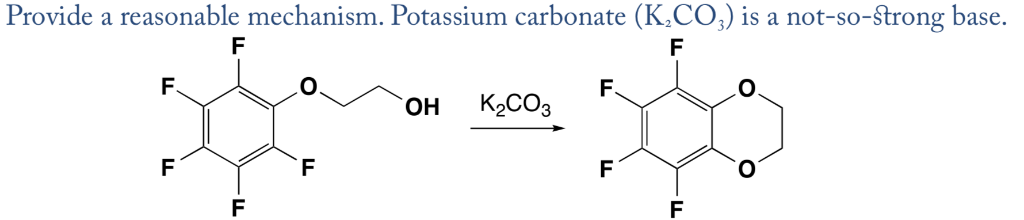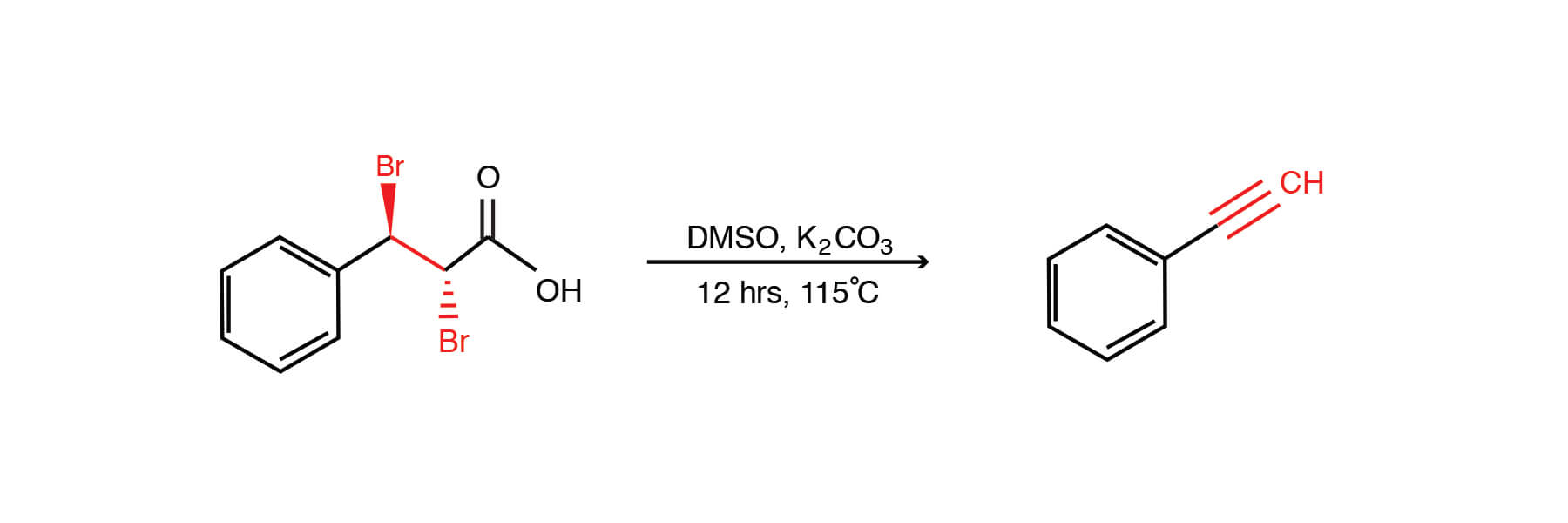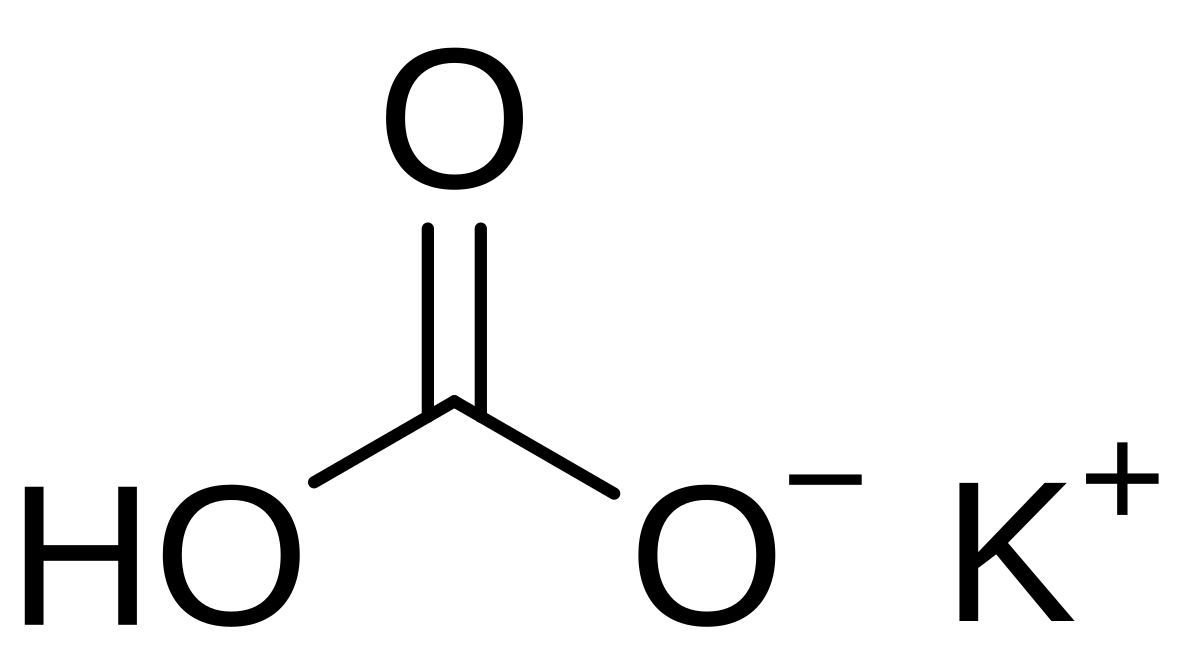
Biodiesel Production from Sunflower Oil Using K2CO3 Impregnated Kaolin Novel Solid Base Catalyst | Catalysis | ChemRxiv | Cambridge Open Engage

K2CO3‐activated Hydrosilylation: from Redistribution of Polymethylhydrosiloxane to Selectively Reduction of Aldehydes and Ketones - Zhao - 2017 - ChemistrySelect - Wiley Online Library
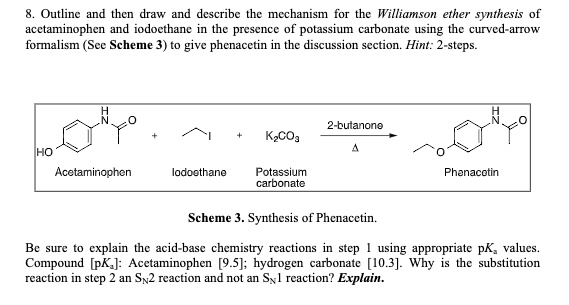
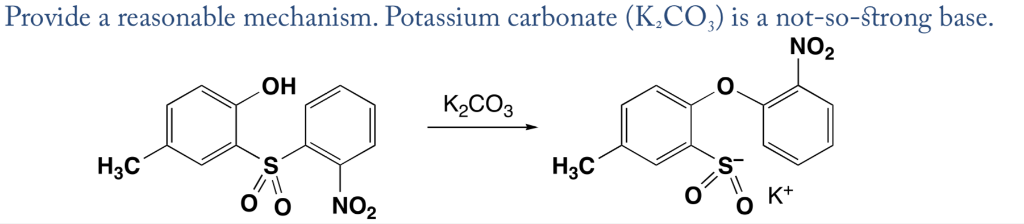
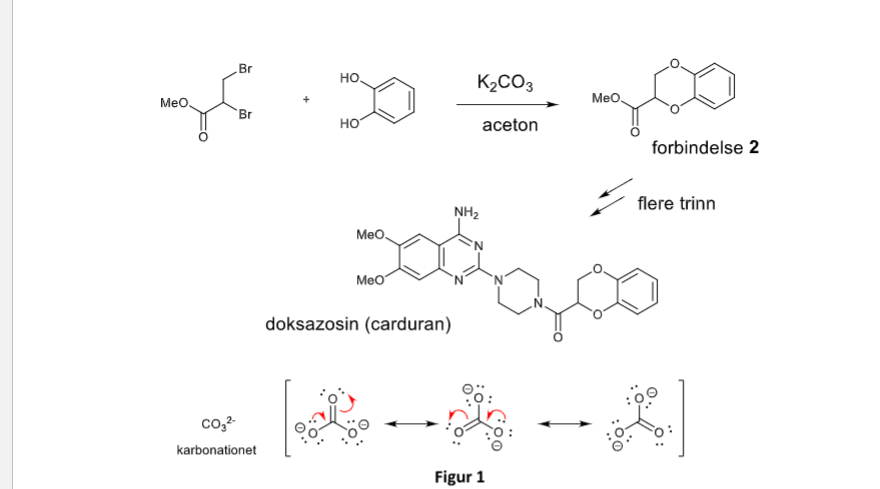
SOLVED: Outline and then draw and describe the mechanism for the Williamson ether synthesis of acetaminophen and iodoethane in the presence of potassium carbonate using the curved-arrow formalism (See Scheme 3) to

Potassium carbonate as a base for the N-alkylation of indole and pyrrole in ionic liquids - ScienceDirect
Methods for synthesizing diethyl carbonate from ethanol and supercritical carbon dioxide by one-pot or two-step reactions in the
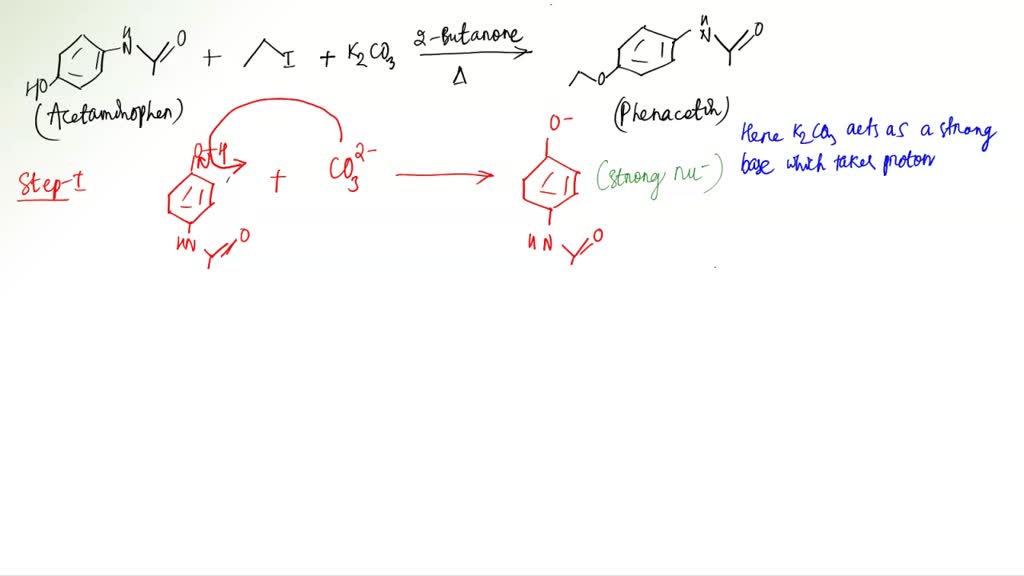
SOLVED: Outline and then draw and describe the mechanism for the Williamson ether synthesis of acetaminophen and iodoethane in the presence of potassium carbonate using the curved-arrow formalism (See Scheme 3) to

Proposed schematic illustration of the K2CO3-Gly DES formation mechanism | Download Scientific Diagram
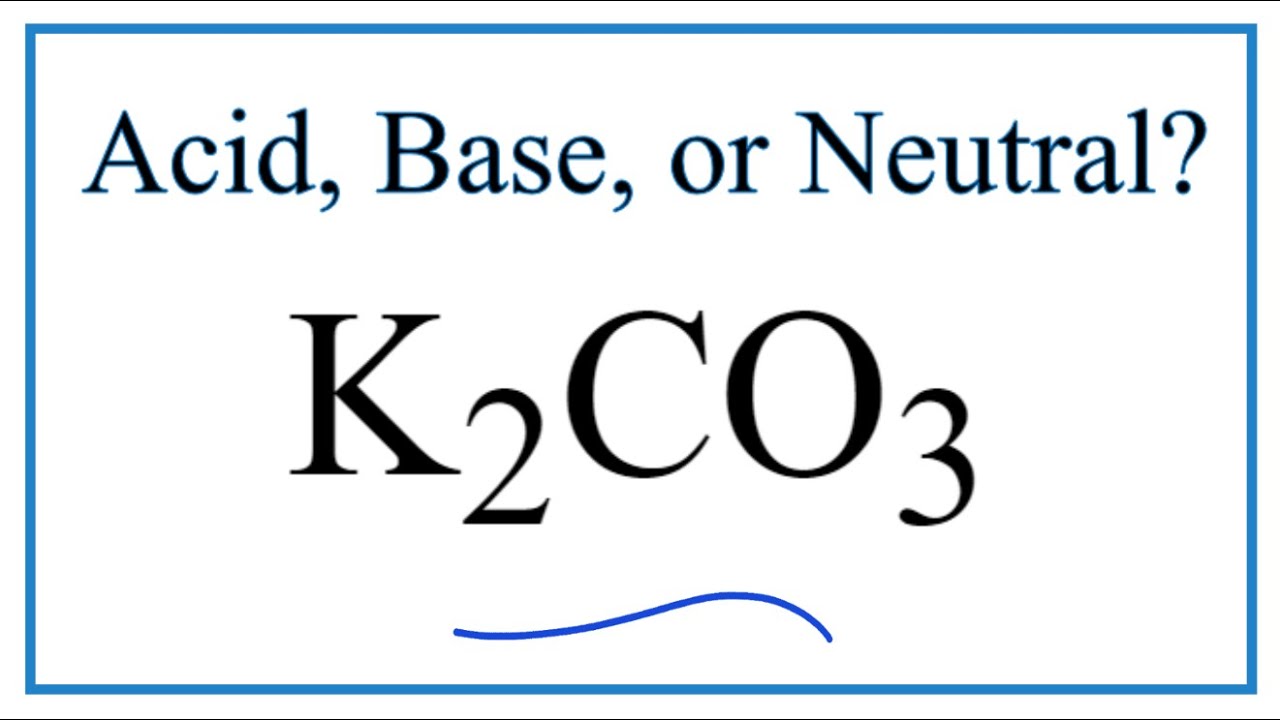
32. Nature of K2CO3 is basic because 1. K+ react with water 2. CO32 react with water 3.k+ and CO32 react with water 4.NONE OF THESE

A K2CO3‐Mediated Regioselective Synthesis of Indole/Pyrrole‐Fused 1,4‐Oxazines: An Unexpected Indole‐Fused Azlactone Synthesis - Vandavasi - 2014 - European Journal of Organic Chemistry - Wiley Online Library

Catalytic Hydroboration of Aldehydes, Ketones, and Alkenes Using Potassium Carbonate: A Small Key to Big Transformation | ACS Omega