GDM in the form of a DBN. The solid lines define the basic structure;... | Download Scientific Diagram
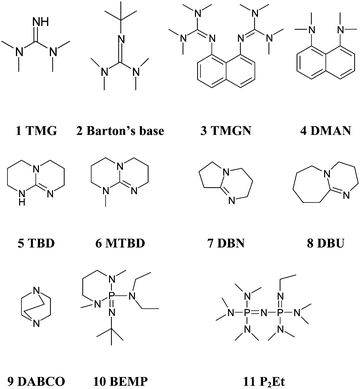
Investigating the Underappreciated Hydrolytic Instability of 1,8-Diazabicyclo[5.4.0]undec-7-ene and Related Unsaturated Nitrogen

The basic structures of the restricted Boltzmann machine and the DBN model. | Download Scientific Diagram
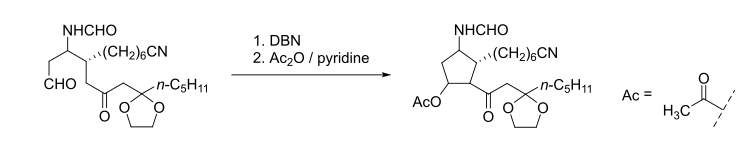
Catalysts | Free Full-Text | Organic Base-Catalyzed C–S Bond Construction from CO2: A New Route for the Synthesis of Benzothiazolones
Investigating the Underappreciated Hydrolytic Instability of 1,8-Diazabicyclo[5.4.0]undec-7-ene and Related Unsaturated Nitrogen
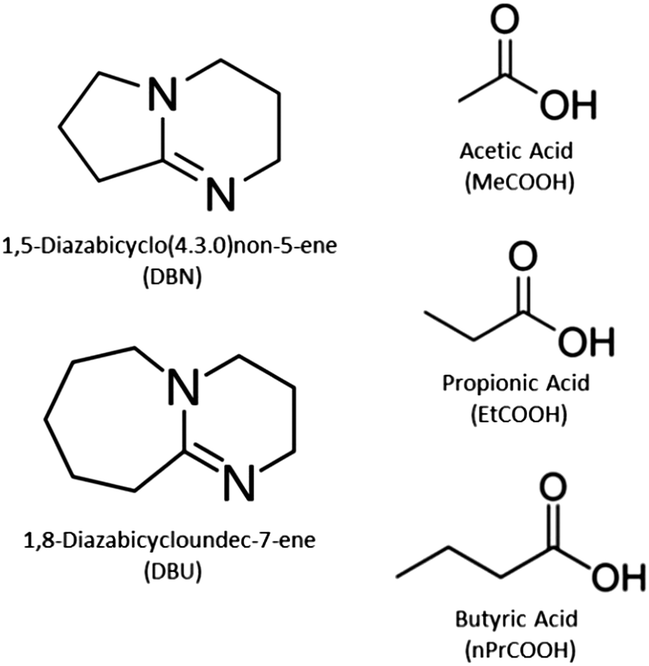
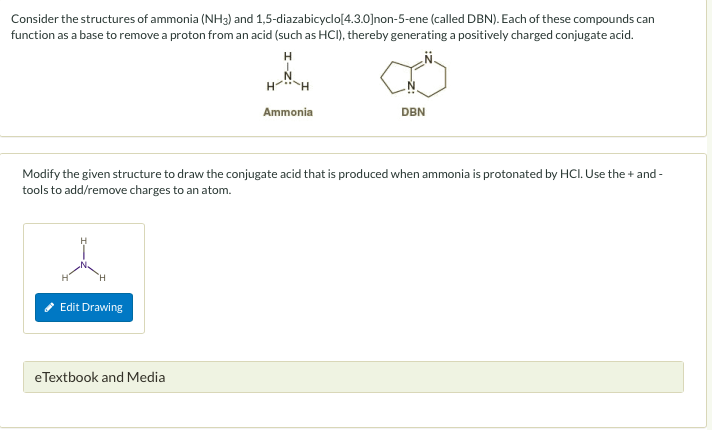
Vaporization of protic ionic liquids derived from organic superbases and short carboxylic acids - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C7CP02023F